Red Planet’s interior may not churn much
An enduring source of magma on Mars fueled volcanic eruptions for billions of years, clues inside a rock flung from the Red Planet reveal.
The newfound rock belongs to a batch of meteorites called shergottites that originated from the same Martian volcanic system, researchers report February 1 in Science Advances. But the new rock is considerably older than its counterparts. While previously discovered shergottites solidified from Martian magma between 427 million and 574 million years ago, the new rock formed around 2.4 billion years ago, chemical analyses show.
Such a wide range of ages means that a volcanic system on Mars churned out hot rocks from a stable source of magma for nearly half of the planet’s history, says study coauthor Thomas Lapen, a geologist at the University of Houston. That endurance could help scientists better understand Mars’ interior. “These are some of the longest-lived volcanoes in the solar system,” Lapen says.
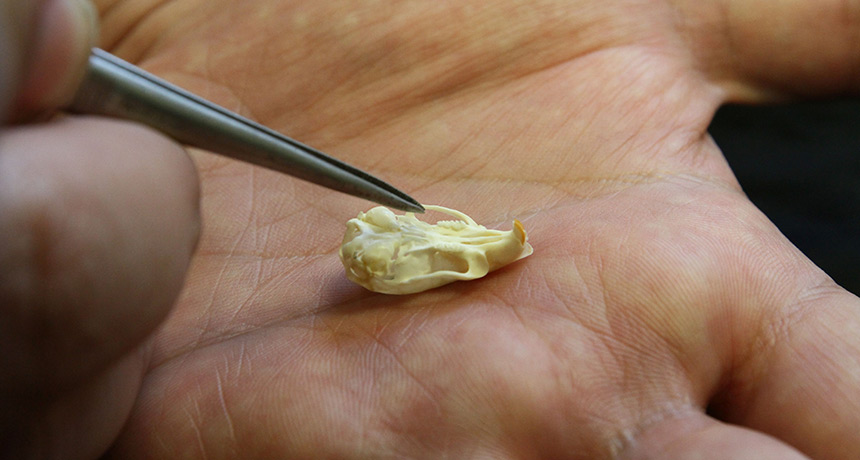
Lapen and colleagues studied elements inside a Martian meteorite discovered in Algerian desert in 2012. Some of those elements serve as stopwatches that record the history of the rock. Isotopes of beryllium and aluminum, formed during exposure to cosmic rays, reveal that the rock zipped through space for around 1 million years. The steady decay of carbon 14 — left behind after cosmic ray collisions — suggests that the rock landed on Earth roughly 2,300 years ago. By combining these two measurements, the researchers found that the meteorite probably blasted off Mars alongside other shergottites a little over a million years ago. This exodus probably followed a massive impact in Mars’ volcano-filled Tharsis region.
The rocks share more than their exit route, the researchers found. Chemical similarities between the meteorites suggest that they all originate from the same source of hot rock deep within the Red Planet. That’s surprising given that the mix of radioactive elements inside the newfound meteorite suggests it solidified 1.8 billion years earlier than the next oldest shergottite, Lapen says.
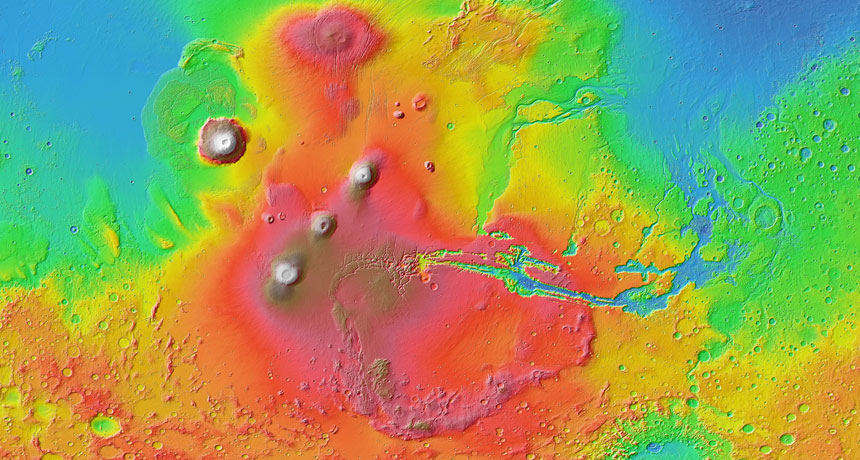
Mars is known to have many volcanic systems across its surface, all fed by magma upwelling from the planet’s depths. Studies have previously suggested that some of these systems operated for billions of years. Though little is known about the Martian interior, many scientists had assumed that the magma feeding this volcanism changed over time as the Martian interior mixed. The absence of any difference in composition of the shergottites suggests Mars’ interior is relatively stagnant. That may result from Mars’ lack of plate tectonics, a process that helps blend Earth’s innards, Lapen proposes. Understanding the differences between Earth and Mars could help reveal why the two planets took such different trajectories, with Earth so much more life-friendly than Mars (SN: 5/2/15, p. 24).
Similarities between the shergottites could have another explanation, says planetary scientist Stephanie Werner of the University of Oslo. Large impacts can melt rocks, resetting their age. The shergottites may have formed around the same time billions of years ago before some had their ages altered by impacts over time, she proposes.
Upcoming missions will help illuminate what’s going on beneath the Martian surface, says James Head, a planetary scientist at Brown University in Providence, R.I. NASA’s InSight lander, currently slated for launch in 2018, will use seismic activity to map the Red Planet’s interior.